This application note describes the high-speed analysis of linezolid following the draft guidance of International Harmonization of Pharmacopoeias.
 Linezolid is a new class of synthetic oxazolidinone antibiotics and has demonstrated efficacy against multidrug-resistant strains such as methicillin-resistant S. aureus (MRSA) and multidrug-resistant tuberculosis (MDR-TB). For a successful commercialization of the drug, factors such as quality control, safety and efficiency are key.
Linezolid is a new class of synthetic oxazolidinone antibiotics and has demonstrated efficacy against multidrug-resistant strains such as methicillin-resistant S. aureus (MRSA) and multidrug-resistant tuberculosis (MDR-TB). For a successful commercialization of the drug, factors such as quality control, safety and efficiency are key.
In preparation for the adoption of the International Council for Harmonisation (ICH) guidelines, we demonstrate (a) the analysis of linezolid based on the U.S. Pharmacopeia (USP) and (b) a high-speed analysis based on the draft ICH guideline. The proposed improvements, described in the application, are within the allowable adjustments to the chromatography analytical conditions in the ICH draft, and allow the fast and accurate analysis of linezolid. Both analysis of linezolid was conducted using the Nexera™ Series UHPLC and Shim-pack Scepter C18 (3 µm and 1.9 µm) and showed good resolution, peak shape and precision. For the high-speed analysis, linezolid was fully eluted before 5 minutes and this is key in achieving high efficiency for drug development.
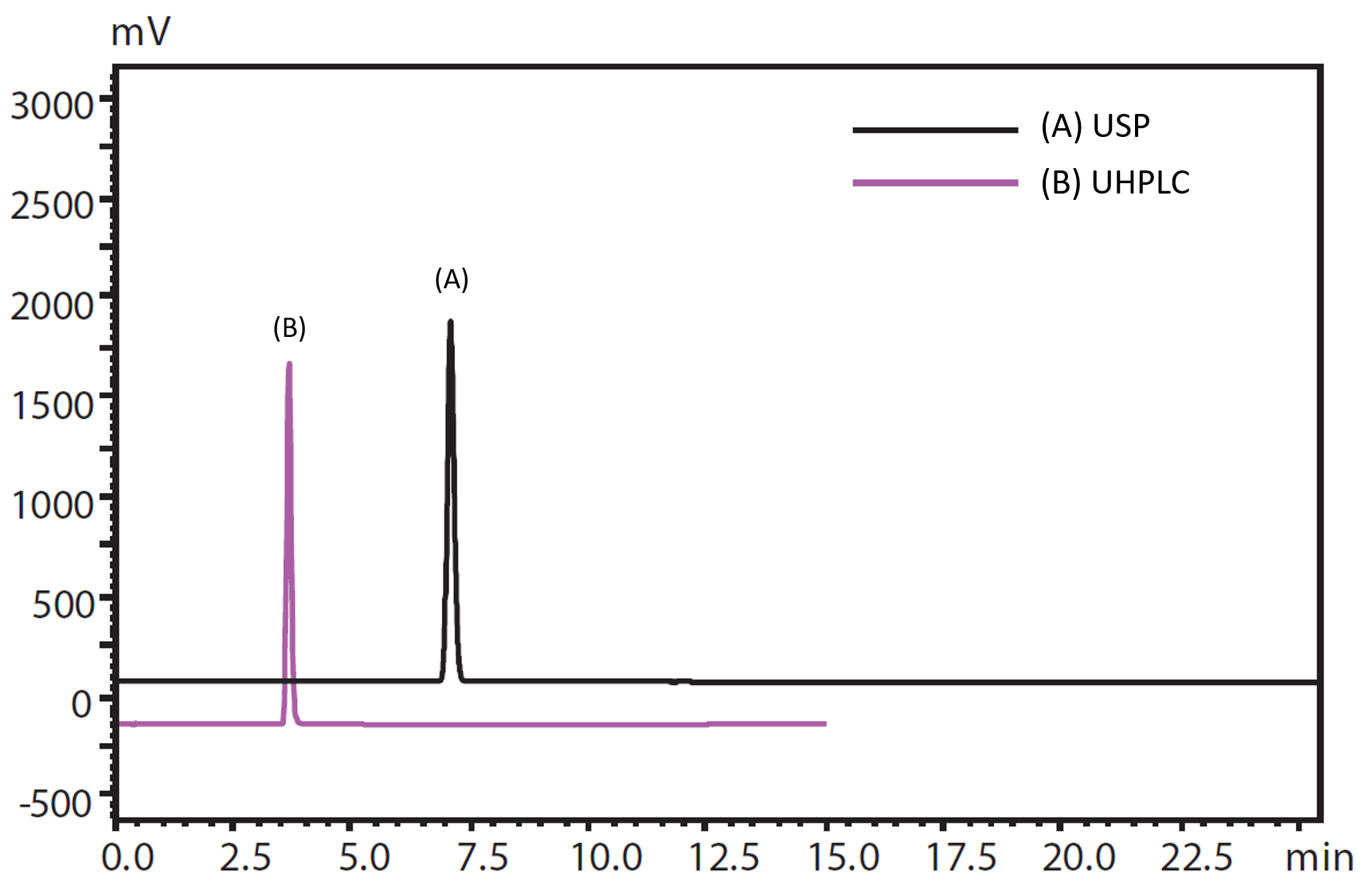
Chromatograms of Linezolid. (A) The analysis of linezolid based on the USP. (B) A high-speed analysis based on the draft ICH guideline.
Download this application note and...
- Understand the allowable range of changes in analytical conditions for the analysis of linezolid
- Readily perform high-speed analysis of linezolid with Nexera™ Series UHPLC and Shim-pack Scepter C18 in under 5 minutes.
