This application note demonstrates a method for the high-speed analysis of pramipexole following the draft guidance of International Harmonization of Pharmacopoeias. It uses Shimadzu's integrated LC system, the Nexera™ - i MT.
 Pramipexole is a dopamine agonist drug used to treat Parkinson’s disease. The current pharmacopeias specify the use of gradient elution as a test method. In contrast, ultra-high performance liquid chromatography (UHPLC) is becoming a popular technology in the pharmaceutical field, with improved cost-effectiveness, throughput and analysis time. It is anticipated that revalidation will be required for high-speed UHPLC analysis to be adopted.
Pramipexole is a dopamine agonist drug used to treat Parkinson’s disease. The current pharmacopeias specify the use of gradient elution as a test method. In contrast, ultra-high performance liquid chromatography (UHPLC) is becoming a popular technology in the pharmaceutical field, with improved cost-effectiveness, throughput and analysis time. It is anticipated that revalidation will be required for high-speed UHPLC analysis to be adopted.
To facilitate seamless transfer of analytical conditions found in pharmacopeia to UHPLC, the Method Transfer tool for Shimadzu Nexera™-i MT can be used. In this application news, we demonstrate how Nexera™-i MT can transfer the gradient profile and flow-rate from a current USP method into a new UHPLC method. 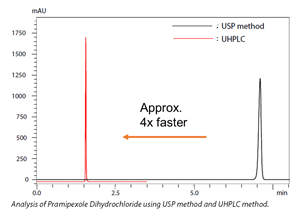 The calculation algorithm is constructed based on the draft guidance of international harmonization guidelines. The algorithm is used for the method transfer and creation. Using the UHPLC method deduced from the algorithm, the analysis results of pramipexole dihydrochloride met all the criteria of the system suitability test. Furthermore, analysis using the UHPLC method demonstrated reduced mobile phase consumption, low sample injection volume and higher sensitivity LC-UV signal/response.
The calculation algorithm is constructed based on the draft guidance of international harmonization guidelines. The algorithm is used for the method transfer and creation. Using the UHPLC method deduced from the algorithm, the analysis results of pramipexole dihydrochloride met all the criteria of the system suitability test. Furthermore, analysis using the UHPLC method demonstrated reduced mobile phase consumption, low sample injection volume and higher sensitivity LC-UV signal/response.