Continuing the previous discussion of the most popular GC detector, the FID, we cover deviations from typical unit carbon response, optimization and troubleshooting.
Deviations from unit carbon response
In the previous article in this series, we introduced the concept of unit carbon response – the ability of an FID to respond proportionally to both the number of carbons in a molecule and the total mass of component in a sample (linear mass response). We also explained some of the flame chemistry involved.
Through the combustion process, hydrocarbons are oxidized in the flame, eventually forming CO2 and H2O. The charged intermediate species (primarily +CHO) give rise to the FID response. If a molecule already incorporates some oxygen, it is further along the oxidation process and will yield a lower proportion of ions and therefore generate a lower response per molecule or mass. This means that the same amount of an oxygenated compound will yield a lower peak of less area than would its saturated counterpart. In a similar fashion, the more oxygen per molecule, the less the response.
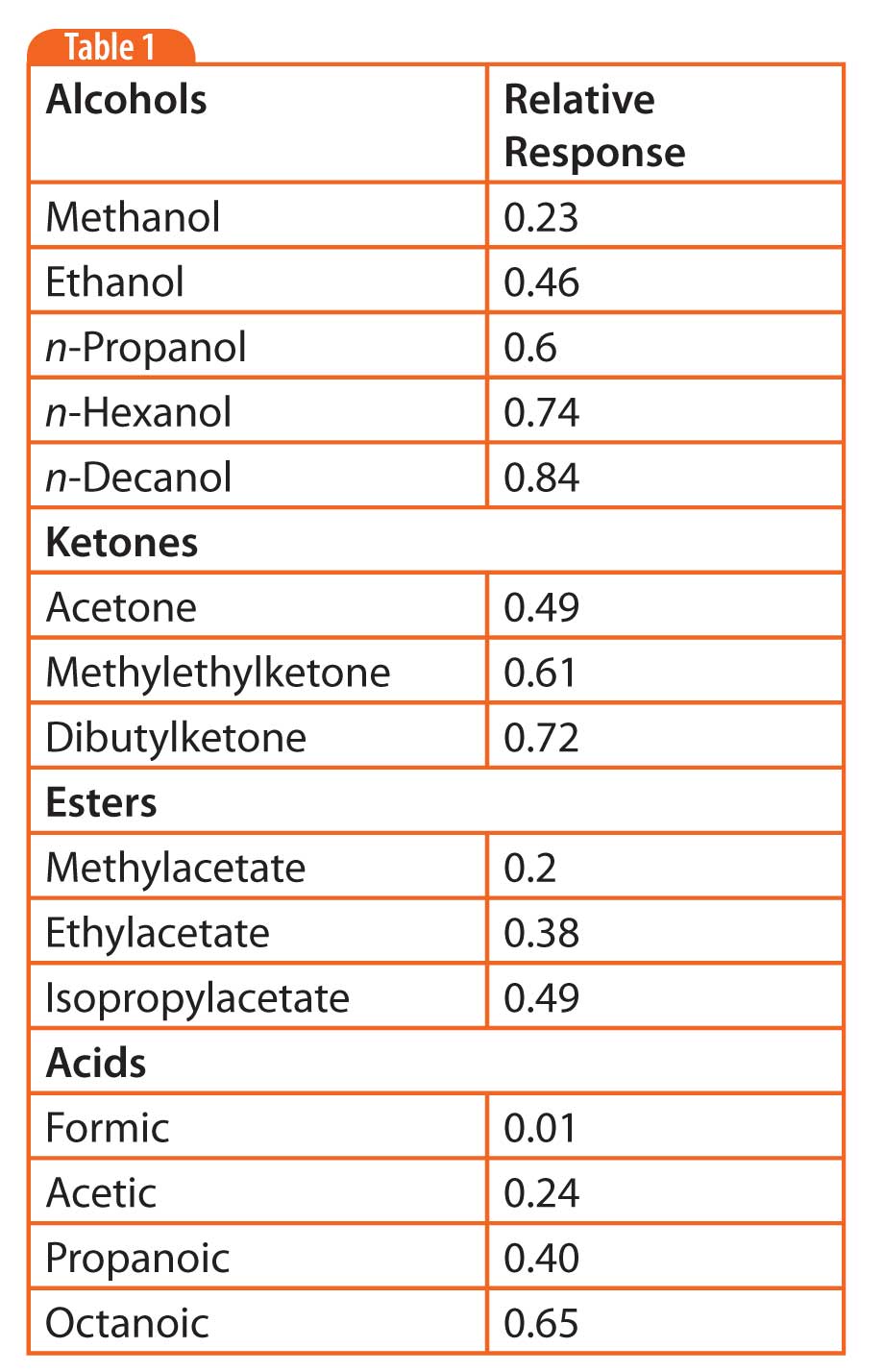
Table 1: Relative FID response of oxygenated compounds.
Response for oxygenated compounds generally decreases in the following order: alcohols, ethers, aldehydes, ketones > esters > acids. The deviation from unit carbon response becomes less significant, the more carbons are in a molecule (the lower the proportion of oxygen per total carbon). Table 1 shows the trend of response for homologous series of oxygenated compounds.
Deviations from unit carbon response are only an issue if one does not calibrate peak responses of individual components. For example, if one were to use an area percent report as a gauge of relative amounts of sample components, then oxygenated compounds would be under reported.
When using uncalibrated reports from an FID to estimate weight percent composition, one must also keep in mind that even when a compound generates close to theoretical response per mass of carbon in the molecule, the molecular mass of the component may be much higher, especially if there are a significant number heteroatoms. This would lead to under reporting of the mass percent of these compounds. For example, one might get a similar response from and equal number of molecules of ethane and dichloroethane, but a 50/50 molar mixture of those gases would actually contain 77 wt% dichloroethane (molecular weights of ethane = 30, dichloroethane = 98). Stated another way, the peak size of ethane for equal masses of dichloroethane an ethane would be approximately 3.3 times larger than that for dichloroethane.
Other functional groups such as nitrogen and sulfur can also cause a less-than-theoretical carbon response. All that being said, for the majority of compounds, FID responses are close enough to accurately estimate composition of individual components within 10% of their actual amounts. This is a unique and powerful attribute of the FID.
Optimization
There is some fine tuning in detector gas flows that may be beneficial in certain circumstances. There is a trade-off between reaching the lowest detection limits and being able to quantitatively analyse high concentration components. The higher the flows of H2 and Air, the more capable the FID is of handling solvent peaks and components of high concentrations. The lower the gas flow, the lower the background noise, and the better the detection limits. Based on the needs of a given analysis, one can adjust the flows accordingly. I recommend keeping the ratio of H2 to Air constant when changing overall flow rates to meet changing application needs.
Optimization troubleshooting
As the FID is sensitive to all carbon containing compounds, it is unfortunately also an excellent detector of contaminants in fuel gases and carrier gas. That is why it is important to use purification traps (usually molecular sieves and/or carbon) on all gases to ensure that background levels of hydrocarbons are low. No matter how well you clean up fuel gases, however, the higher the flow of a gas, the more background contamination will be flowing through the detector and the higher the background signal (higher chemical noise). So if you need to measure near the detection limit, then reducing the fuel gas flows will reduce the noise and you should get better detection limits.
To see how much background noise is coming from fuel or makeup gas contamination, simply monitor the FID signal as you increase or decrease each gas flow by 50% or so. If you do not see much change, then the tested gas and its connecting lines are relatively clean. If you see a dramatic proportional change in signal, then you have identified a source of background and have a choice to make; clean up the gas one way or another, or accept the higher level of noise and inferior detection limits.
You might lower the gas flows in order to minimize the background noise level, but you run when the solvent peak or a high concentration analyte comes through, it will not be completely combusted and risks blowing out the flame and/or contaminating the jet. As a result, peak area or height will not be accurate and you risk affecting subsequent analysis because of a contaminated FID jet. A contaminated jet becomes the source of noise (random sharp spikes) and the flame will become unstable and increasingly prone to flameout. For this reason, it is prudent to ensure gases are clean and to operate at recommended gas flows, especially when high levels of analyte (e.g., mg levels or above) are reaching the detector.
If there is evidence that the FID jet is dirty (spikes, easy flame out when a solvent peak elutes), then the jet should be removed and cleaned with a fine wire or it should be replaced.
Another potential source of potential jet contamination is from graphite ferrules that are typically used to connect columns. Even though they make convenient and reliable connections, graphite ferrules are soft and can extrude and flake off into the FID jet. These flakes/particles can make their way to the top of the jet where they cause peak tailing and increased detector noise. So, be careful to not over tighten graphite ferrules. If you suspect contamination, remove and clean or replace the jet.
References
1. W.A. Dietz, J. Gas Chromatogr. , 5 (1967) 68.
2. J.T. Scanlon, D.E. Willis, J. Chrom. Sci. , 23 (1985) 333-340 and references therein.
This blog article series is produced in collaboration with Dr Matthew S. Klee, internationally recognized for contributions to the theory and practice of gas chromatography. His experience in chemical, pharmaceutical and instrument companies spans over 30 years. During this time, Dr Klee’s work has focused on elucidation and practical demonstration of the many processes involved with GC analysis, with the ultimate goal of improving the ease of use of GC systems, ruggedness of methods and overall quality of results. If you have any questions about this article send them to techtips@sepscience.com
