In this article we continue the previous discussion of ferrules used in gas chromatography.
Ferrules of graphite, Vespel® (polyimide), Vespel®-graphite mixtures, and Teflon® (polytetrafluoroethlyene, PTFE) will be discussed. Even though graphite is not a polymer, its characteristics are more similar to the synthetic polymers than metal ferrules discussed last month, so it is included. Attributes of these ferrule materials are summarized in Table 1.
Graphite and polymeric ferrules have several advantages for use in gas chromatography over metal (especially hard metal) ferrules:
- They can seal against imperfect surfaces with little force
- They can be used with virtually any type of tubing or column including glass and fused silica
- They can also be hand drilled with a pin vise to get the right size for any given tube or column.
However, because they are organic and porous, graphite and polymeric ferrules do have some general weaknesses that constrain their uses as well:
- Polymers have a limited temperature range (compared to metal ferrules)
- They are more permeable to air infiltration (a function of polymer density)
- They sometimes come out of their commercial packaging, or the lab drawer, contaminated. They are then a source of ghost peaks and baseline disturbances. Contamination comes from poor manufacturing processes as well as poor choices in packaging (the contamination comes from the packaging).
- They can interact with sample components or solvent causing tailing or losses, especially at trace levels

Figure 1: Examples of graphite and polymeric ferrules.
Graphite has been a favorable ferrule material for capillary column use from the beginning of gas chromatography. It is very forgiving because it is so soft and can deform and seal in almost any space. Graphite is easy to identify because it can be deformed by pinching with your fingers, or scratched easily with your fingernail. I have successfully used the wrong size ferrule (because I ran out of the right size) and was able to either easily expand the hole to accommodate a larger column or to compress a larger ferrule just by putting it on the column and tightening the fitting a little more than usual. The graphite reformed in both cases to create a seal. However, this malleability can lead to one of the biggest problems with graphite ferrules as well. This ability of graphite to reform can cause it to extrude through openings in fixtures into the neighboring spaces. Then, graphite pieces end up contaminating areas like the bottom of the inlet or the detector jet. These graphite pieces can interact with sample causing losses, tailing, and can become a constant source of contamination (graphite acts as a “chemical sponge”).
A second major problem with 100% graphite ferrules is that they are very permeable to air. So, when using air sensitive columns (e.g., carbowax) or detectors (e.g., mass spectrometers or ECDs) one should choose something else.
Some inlet and detector designs use graphite ferrules wherein the graphite is contained within a secondary metal tube. This provides the benefits of graphite ferrule material, while greatly addressing the issues of deformation, extrusion, and infiltration of air.
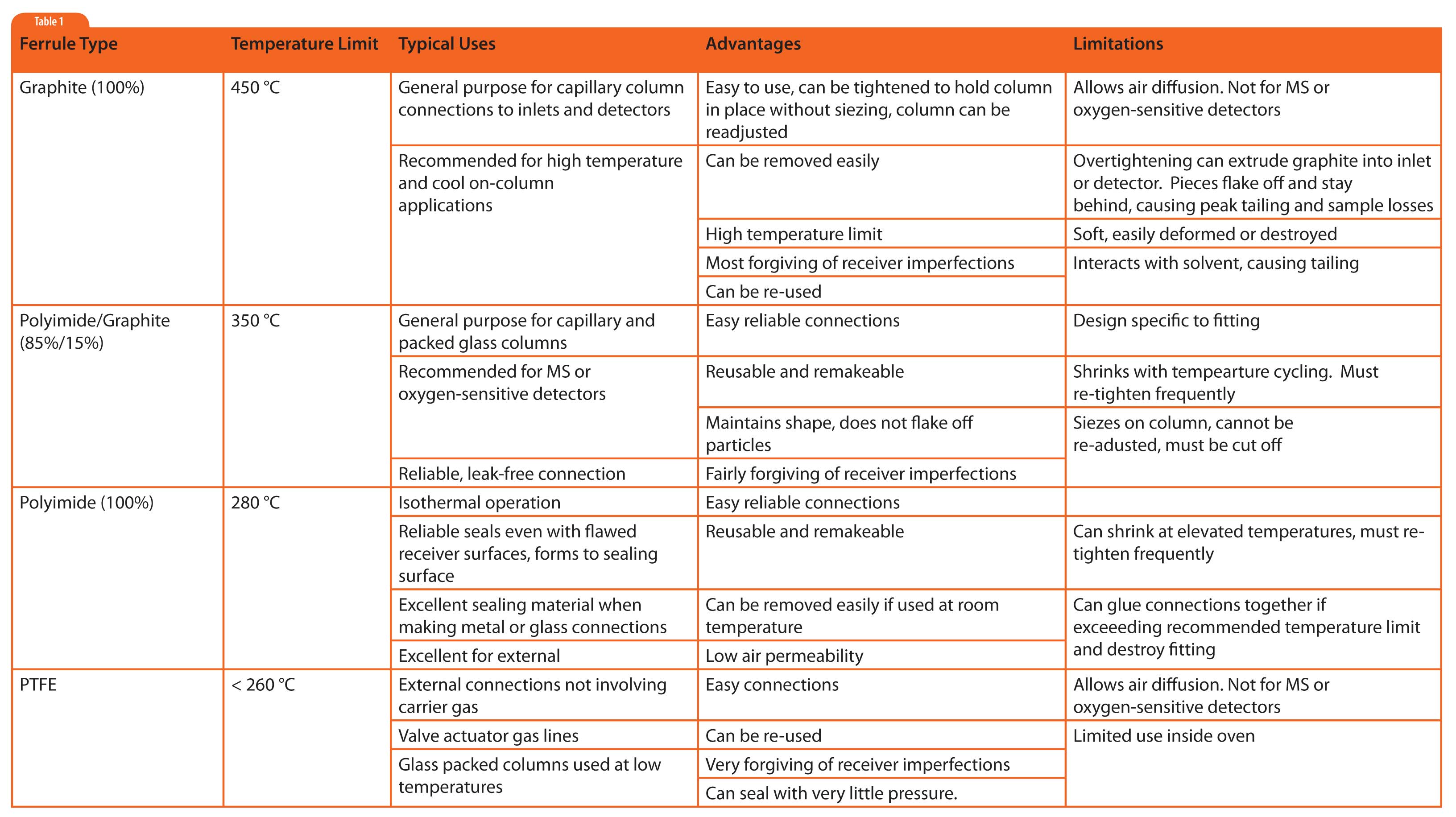
Table 1: Summary of attributes for graphite and polymeric GC ferrules.
Polyimide is a polymer with high temperature stability and low outgassing. Because of its relatively high temperature stability, polyimide is the coating of choice for the outside of fused silica columns. Polyimide ferrules are easy to identify because they are brown (see Figure 1). Polyimide is fairly rigid and can be molded into shapes that match fixtures, fittings, and devices typically designed for metal ferrules. Even though it has high temperature stability, polyimide is very sticky when heated, so it is not a good choice in high-heat zones. It can seize on the tube and in worst-case scenarios, it sticks inside the fitting. For this reason, 100% polyimide ferrules find most use outside the GC oven. I prefer them over metal ferrules for room temperature or low temperature connections since they are much more forgiving than metal ferrules when sealing against flawed tubing, they can be removed without having to be cut off the tubing, and they have low air permeability.
The stickiness of polyimide can be greatly reduced by adding a small amount of graphite (15% is common). The mixture retains the benefits of:
- Being able to be molded into desired shapes
- High temperature stability
- Ferrule does not extrude when tightening
- Can seal against imperfect surfaces
- Has low air permeability
- Soft enough for use with fused silica
Because of these benefits, graphite/polyimide ferrules are the dominant ferrule type when making fused silica column connections in the GC oven. graphite/polyimide ferrules do tend to shrink with temperature cycling, so they need to be retightened several times (after cool down) within the first 10 temperature programmed runs or so.
PTFE ferrules are very inert and have minimal interaction with samples and solvents. They are very easy to seal, often requiring only hand tightening. When disconnecting fittings, PTFE fittings slide right off the tubing and can be re-used multiple times. However, they have the most restricted upper temperature limit and are somewhat permeable to air, so use inside the GC oven is quite limited. PTFE ferrules are used primarily for low temperature applications requiring the most inert connections, and for tubing connections outside the oven when one wants reliable, easily adjustable and removable connections. PTFE fittings are often used for compressed air supply lines for valve actuators.
This blog article series is produced in collaboration with Dr Matthew S. Klee, internationally recognized for contributions to the theory and practice of gas chromatography. His experience in chemical, pharmaceutical and instrument companies spans over 30 years. During this time, Dr Klee’s work has focused on elucidation and practical demonstration of the many processes involved with GC analysis, with the ultimate goal of improving the ease of use of GC systems, ruggedness of methods and overall quality of results. If you have any questions about this article send them to techtips@sepscience.com
