This application note will help you understand how trap-free 2D LC-MS allows online conversion of non-volatile to volatile mobile phase conditions and how the LCMS-9030’s high mass accuracy can be used to conduct structural analysis of impurities.
 Impurity profiling is crucial for quality control in pharmaceuticals. These unwanted substances, remain or are generated during drug formulation and are present together with the active pharmaceutical ingredient (API), thereby affecting the safety and quality of drugs. The APIs and identified impurities are commonly determined using HPLC-UV while unknown impurities are identified using mass spectrometry (MS) techniques; the latter provides better sensitivity and specificity for impurity identification. MS is typically conducted as a separate analysis under different conditions.
Impurity profiling is crucial for quality control in pharmaceuticals. These unwanted substances, remain or are generated during drug formulation and are present together with the active pharmaceutical ingredient (API), thereby affecting the safety and quality of drugs. The APIs and identified impurities are commonly determined using HPLC-UV while unknown impurities are identified using mass spectrometry (MS) techniques; the latter provides better sensitivity and specificity for impurity identification. MS is typically conducted as a separate analysis under different conditions.
In this article, an improved workflow for structural analysis of impurities in the atorvastatin calcium solution was demonstrated using a trap-free 2D HPLC system coupled with Q-TOF MS/MS. With this system, the API (atorvastatin) and impurities were first detected using HPLC-UV under non-volatile mobile phases. For further structural identification of impurities, the mobile phases were converted to volatile conditions online for a seamless QTOF MS/MS analysis. This automatic and efficient setup gives the same elution order of analytes, prevents any overlooking of impurities and allows the accurate determination of API and high-confidence identification of impurities.
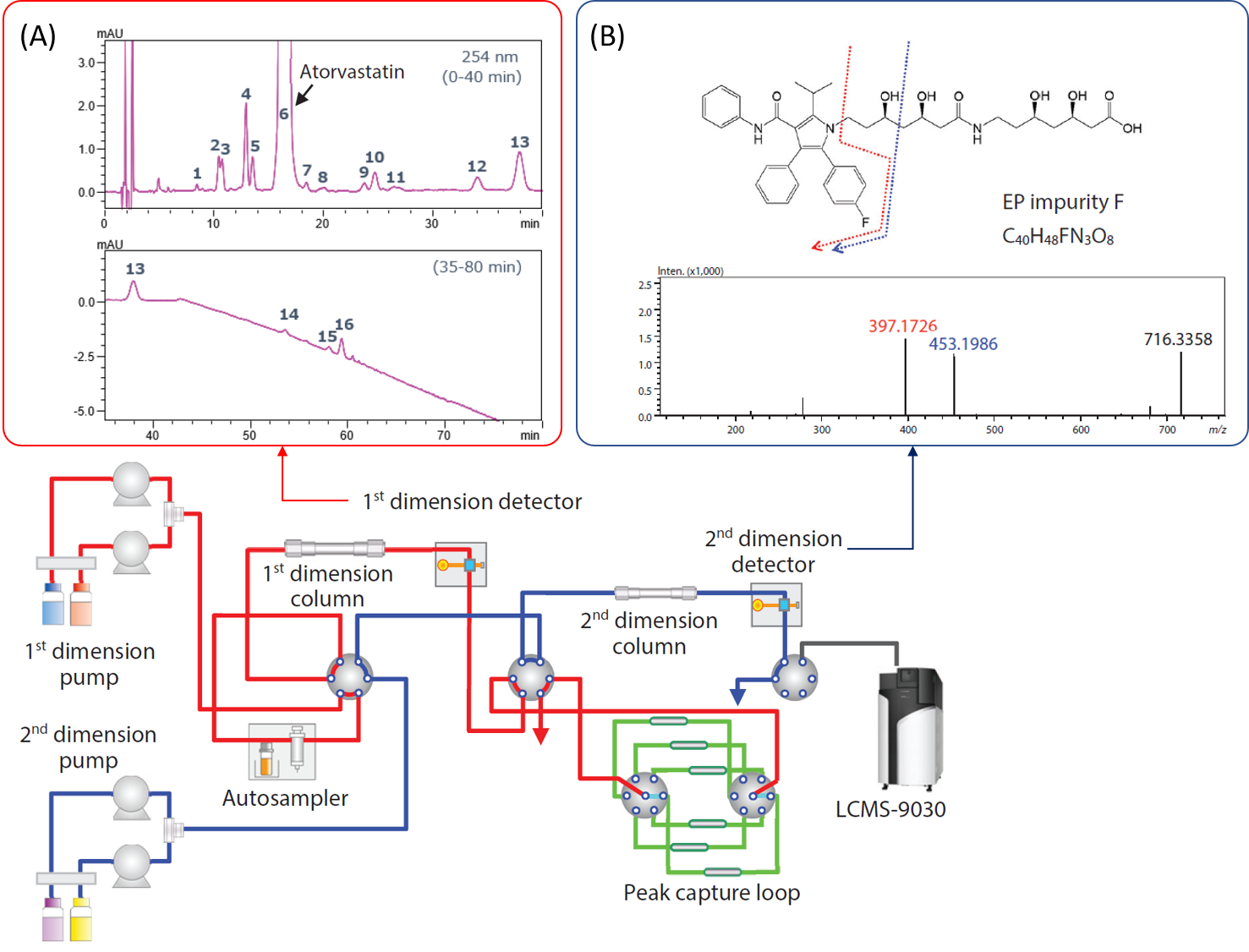
2D-LC-MS/MS QTOF Configuration. (A) 1st dimension – HPLC-UV results (B) 2nd dimension – Structural identification of impurity.
Download this application note and...
- Understand how trap-free 2D LC-MS allows online conversion of non-volatile to volatile mobile phase conditions
- Understand how the LCMS-9030’s high mass accuracy can be used to conduct structural analysis of impurities.
