Shimadzu has produced an application note describing a newly developed method for the high-speed analysis of pharmaceuticals and related substances in compliance with European Pharmacopoeia using the Nexera-i MT.
 Introduction
Introduction
According to "Adjustment of chromatographic condition" described in the 8th edition of the European Pharmacopoeia (EP), parameters in TLC, LC, GC and SFC may be adjusted without revalidation provided that the system suitability requirements are satisfied. For example, reduction in column particle size by 50% is within the permitted range for isocratic elution.
This article introduces an example wherein high speed analysis of pharmaceuticals and related substances was newly developed in compliance with the EP, using the Nexera-i MT integrated high performance liquid chromatograph. Since the Nexera-i MT integrates both HPLC and UHPLC flow lines, it allows method modification to be conducted in a single system, and the new method may be adopted without transferring it to a new system, thus minimizing the work required improving productivity.
 Allowable Adjustment Range of HPLC
Allowable Adjustment Range of HPLC
The LC section in "Adjustment of chromatographic condition" is broadly classified into isocratic elution and gradient elution. For gradient elution, the allowable adjustment range of methods differs from that of isocratic elution because peak-shifting caused by unstable gradient profile of the mobile phase can lead to misidentification and overlapping of multiple peaks. Furthermore, in the case of gradient elution, it is stated that the elution time of the principal peak must be within 15 % of that in the testing method. Thus, the adjustments of many parameters are restricted for gradient elution and further high speed analysis is practically impossible. Therefore high speed analysis can only be achieved for isocratic elution.
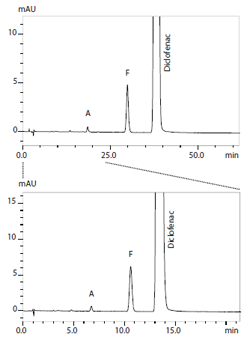
High Speed Analysis of Ivermectin, Diclofenac Sodium and Related Substances
Ivermectin, belonging to macrolides, is known as a therapeutic drug for strongyloidiasis, an antiscabietic and an antiparasitic agent for animals. Diclofenac is widely used as an antipyretic and a painreliever. In this application note an example of high speed analysis of both ivermectin and diclofenac sodium and related substances based on the EP is presented.