Shimadzu has produced an application note presenting data obtained from Class 1 and Class 2 standard solutions, in accordance with Water-Soluble Articles, Procedure A, in USP <467> Residual Solvents.
 Introduction
Introduction
Headspace GC methods specified in the USP (U.S. Pharmacopeia), General Chapters <467> Residual Solvents, are commonly used for analysis of residual solvents. This application note presents data obtained using the Shimadzu HS-20 Headspace Sampler and Nexis GC-2030 Gas Chromatograph, from Class 1 and Class 2 standard solutions, in accordance with Water-Soluble Articles, Procedure A, in USP <467> Residual Solvents.
 Results
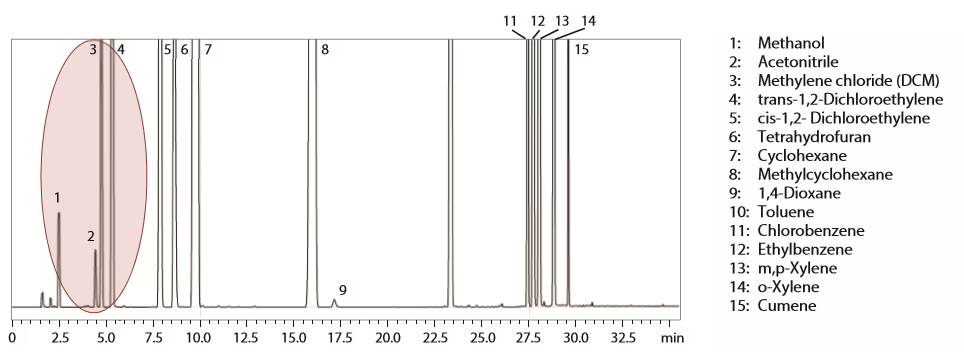
Results
For Class 1 standard solution, the system easily fulfilled the sensitivity requirements including the low-responding carbon tetrachloride. On the other hand, chromatographic resolution of acetonitrile and methylene chloride that is required for Class 2 standard mixture was completely achieved at a resolution of 2.4.
