In previous blog posts we've talked about various aspects of buffers. We saw that some buffers were better than others and that there were certain buffer-preparation practices that should be avoided. This prompted one reader (I.M.) to remind me of another wise practice relating to buffers – to anticipate the use of an LC-UV method with LC-MS detection. This also reminded me of my years as a Boy Scout and scout leader – and that the Boy Scout Motto is “Be Prepared.” This is a good motto for chromatographers, too.
Most of us have spent our lives working with HPLC with a “traditional” optical detector. By this I mean one of the popular UV, fluorescence or refractive index detectors. Because the mobile phase passes unchanged through these detectors, we seldom have concerns about the type of buffer used. Of course, we don’t want to choose a buffer that gives a high background, but as long as we’re using phosphate or acetate, we’re pretty safe on this count. When we used a chemically active detector, such as electrochemical (amperometric), where chemical changes occur in the detector, some extra precautions sometimes were taken, but for the most common detectors, we had little or no concern.
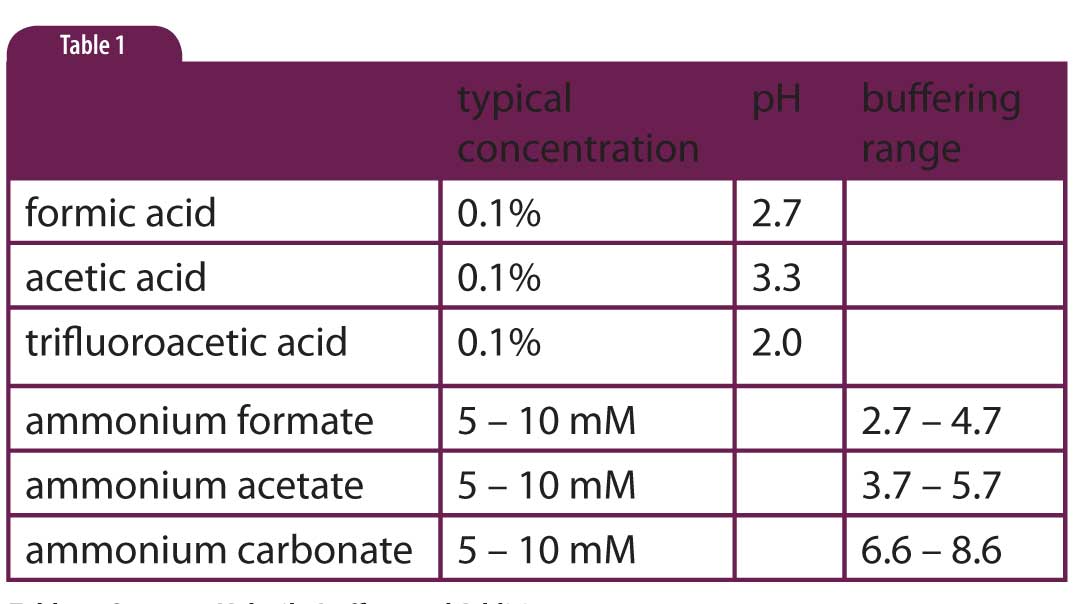 Table 1
Table 1
Over the last couple of decades, the use of the mass spectrometer (MS) as a detector has become increasingly common. At the same time, we also have seen the introduction of two other detectors, the evaporative light scattering detector (ELSD) and the charged aerosol detector (CAD). The LC-MS or LC-MS/MS is the standard detector for the analysis of drugs in biological samples (“bioanalytical” separations), as well as many of the “omics” separations (proteomics, metabolomics, etc.). The ELSD and CAD are gradually displacing the refractive index detector in many cases. All three of these detectors, LC-MS, ELSD and CAD, have one common feature not present in the optical detectors mentioned above. This is the requirement that the mobile phase must be removed by evaporation.
Evaporation of the mobile phase is not a big deal for acetonitrile (ACN) or methanol (MeOH),and with the new interfaces, even a significant percentage of water can be evaporated. But if the buffer or other mobile-phase additive is not volatile, you can end up with a snowstorm in the detector, and you’ll wish you had become a mushroom picker instead of a chromatographer. The obvious solution to this is to use a buffer that is sufficiently volatile that it will evaporate along with the rest of the mobile phase.
In Table 1 lists the most common buffers and additives used for LC-MS. These are equally suitable for ELSD or CAD applications. The key properties are the ability to control the pH of the mobile phase, and being sufficiently volatile to evaporate quickly in the interface between the HPLC system and the detector. You’ll note that sometimes we just add the acid to the mobile phase instead of making a true buffer. For example, 0.1% formic, acetic or trifluoroacetic acid is added. The assumption is that the primary function of the additive is to keep the pH low, not its buffering capability. You will also note in Table 1 that there are gaps in the buffering range of true buffers, notably in the 5.7 < pH < 6.6 region. Unfortunately, I know of no volatile buffers that cover this region, so you’ll just have to adjust ammonium acetate or ammonium carbonate to get the desired pH and realize that the buffering capacity is very low at the selected pH.
Now back to the original suggestion: Be Prepared. As these evaporative detectors become more and more popular, the likelihood increases that one of your methods will need to migrate to one of these detectors, especially LC-MS or LC-MS/MS, at some future time. For example, let’s say you are developing an impurity method for a new pharmaceutical product. For routine use, it is likely that the method will be LC-UV, because such methods tend to be rugged, cover a wide dynamic range, and give exceptional precision and accuracy. However, it is also very likely that you will discover an impurity or unknown peak for which you need to get additional qualitative data. What is it? Is it related to the active ingredient or one of the excipients? The LC-MS can help to answer such questions, but if you developed the method with a phosphate buffer, you can’t just move the method to the MS and hope to get results. First, you must migrate the method to an MS-compatible mobile phase. This may be as simple as switching from phosphate to formate, or it may require much more work if you observe that selectivity changes with the buffer change. It is much better to be farsighted and anticipate the future need. Start the method development process with an MS-compatible mobile phase, then when the extra analytical power is needed, you can just move the column and mobile phase over to the LC-MS and away you go. So be a good Boy Scout and Be Prepared!
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his ongoing research with Lloyd Snyder, resulting in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com
