In this article we discuss the merits and deficiencies of sandwich injection techniques.
Sandwich injection techniques began to get more attention in the era when samples were being injected manually. More recently in the era of autosamplers, many of the current autoinjectors have expanded capability to do a variety of sandwich injection techniques, so analysts might be naturally curious about making use of the features. However, you can make things quite worse by blindly adopting a sandwich technique without careful forethought.
What guidance does the literature provide? Analysts often find themselves trying to make sense out disparate claims in the literature about the efficacy of one technique or another, especially if arguments seem logical. I think one can always find a set of conditions wherein anything will work. Said another way, one can find ways of improving results of a given method by changing certain parameters or techniques. Said yet another way, you can prove anything you want by choosing your sample and conditions correctly. But that doesn’t mean that it is a good idea to use the technique or conditions for anything else other than those samples, instrumentation and conditions. There is entirely too much overgeneralization in the literature based on results from narrowly scoped experiments. Such is the case with discussion of sandwich injections.
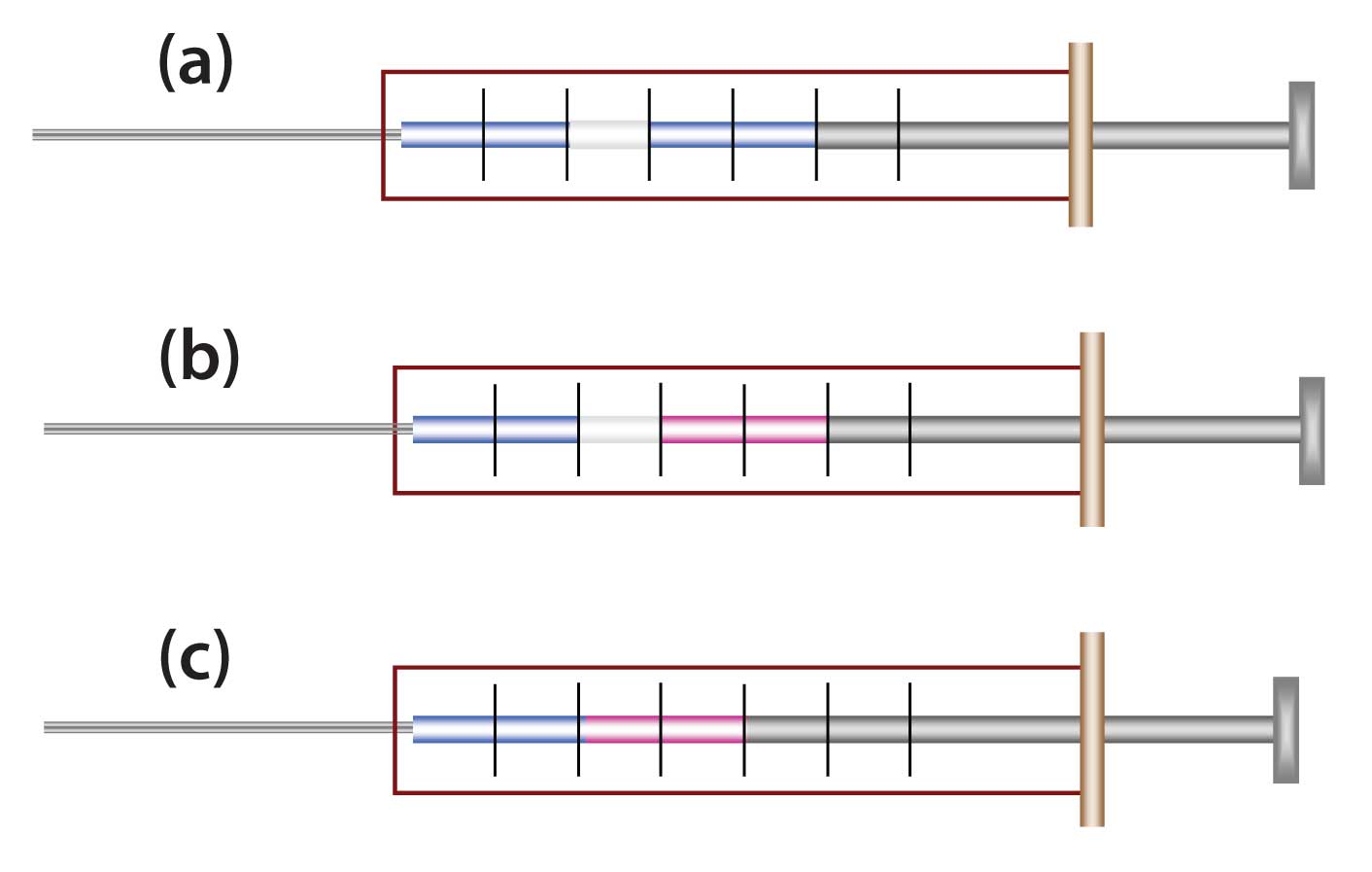
Figure 1: Comparison of some sandwich techniques. (a) Solvent is placed into syringe first, followed by air and then sample. The intent is that the solvent will rinse surfaces and ensure total sample transfer. (b) A different solvent or derivatization reagent is used, separated by air plug. (b) Chasing solvent or reagent in direct contact with sample.
Figure 1 shows a few examples of sandwich injections. Figure 1(a) shows an initial aliquot of solvent followed by air and then sample dissolved in the same solvent type. The air is used to separate the two liquids. In practice the air space usually disappears when the syringe pierces the septum and the contents are exposed to capillary column head pressure.
Figure 1(b) shows use of a different solvent than that of the sample. This has been stated to be of potential advantage when the sample has to be made up in a weaker solvent (e.g., less reactive, cheaper, less toxic). The logic idea is that the stronger solvent might be effective at stripping analytes that otherwise would be retained on the needle surface.
Figure 1(c) is not necessarily a sandwich because there are only two zones, but it is effectively the same as the other two examples in practice; this is a more typically used configuration with autosamplers than with manual injections.
Anyone who has injected a sample into a GC and then pulled the plunger back has seen that some of the sample remained in the needle. It is logical to think that by chasing the sample with solvent, the solvent should force the entire sample out of the needle and then only clean solvent would remain. However, in practice, the process of evaporation from the needle involves solvent and volatile components being preferentially evaporated from the needle and less volatile sample components disproportionately being left behind (i.e., causing high-end needle discrimination).
We have discussed in prior articles that injections into hot inlets are fraught with problems. The problems are influenced by many variables, which is why it is problematic to generalize from one set of conditions or limited optimization space. Some of the variables are listed below. Changing any one of them can change the results.
- Inlet temperature
- Inlet pressure
- Solvent boiling point and molar expansion volume
- Liner volume
- Injection volume
- Liner packing (yes/no, what type, how much, how tight, what position?)
- Total flow rate through inlet
- Injection speed (plunger velocity)
- Needle position in liner during injection
- Type of pneumatic control (flow or pressure, electro-mechanical or mechanical, active or passive, PID control settings, etc.)
- Volume of inlet connection lines (pneumatic capacitance)
- Needle diameter (i.d.)
As the syringe needle proceeds into a hot inlet during manual injections (or slow automated ones), the needle heats and the processes leading to discrimination (distillation leading to quantitative evaporation of low boilers but lagging of high boilers and concentration of high boilers near syringe walls) proceed quickly. By the time the potential washing solvent flows through the needle, it has limited contact with the hot walls and little ability to wash off retained analytes. I have seen little improvement (in general practice) when chasing samples with solvent when doing manual injections. With fast autoinjection, needle discrimination is minimal already so adding more solvent only aggravates problems.
The biggest problem in using sandwich injections is that the amount of solvent doubles (assuming that one uses the same volume of solvent as sample). This can aggravate inlet overload, exaggerating loss of volatiles, degrading repeatability, and contaminating cooler areas of the inlet. The larger volume increases time for analytes to transfer from the inlet to the column and increases the probability of peak splitting and distortion when doing splitless injections because of the higher amount of condensed solvent in the column. Clearly, if one were interested in trying sandwich injections, keen attention must be paid to the many associated variables and one should think careful before changing ANYTHING once a suitable set of conditions is found.
With fast autoinjectors, the residence time in the inlet is much less than 500 msec. This is not possible by manual injection. The less time a needle spends in a hot inlet, the less issues one has. So if one uses a fast autoinjector, many of the original reasons to consider sandwich injections are not relevant; yet the potential incremental problems remain or may actually be aggravated. The same holds true with temperature programmable inlets (i.e., PTVs). A major advantage of PTVs is that sample can be introduced at temperatures below the boiling point of the solvent, thereby eliminating needle discrimination and, therefore, the motivation to consider using a chasing solvent.
An area where I think sandwich injections warrant special consideration (even with fast autoinjectors and PTVs) is intra-inlet derivatization. Figure 1(b) can also be viewed as either preceding or following a sample aliquot with an aliquot of derivatizing reagent. Many derivatizing reactions are fast enough to happen in the time frame of injection and transfer of sample to the column. This approach can be a nice time and money saver for those sample types that are amenable to the technique. Of course the potential problems associated with inlet overload remain and conditions should be carefully optimized (and then not changed).
This blog article series is produced in collaboration with Dr Matthew S. Klee, internationally recognized for contributions to the theory and practice of gas chromatography. His experience in chemical, pharmaceutical and instrument companies spans over 30 years. During this time, Dr Klee’s work has focused on elucidation and practical demonstration of the many processes involved with GC analysis, with the ultimate goal of improving the ease of use of GC systems, ruggedness of methods and overall quality of results. If you have any questions about this article send them to techtips@sepscience.com
