Separation Science, in collaboration with Shimadzu, offers a series of application notes providing solutions for oligonucleotide drug analysis. Click on the button below to download your personal copies.

Thermal Stability Analysis of Nucleic Acid Drugs by TMSPC™-8 Tm Analysis System Thermal stability analysis (Tm analysis) of a nucleic acid was conducted using a Shimadzu UV-1900i ultraviolet-visible (UV-Vis) spectrophotometer and TMSPC-8 Tm analysis system. This system provides a simple method to calculate the Tm value by measuring the absorbance transition at 260 nm at increasing temperatures.
Thermal stability analysis (Tm analysis) of a nucleic acid was conducted using a Shimadzu UV-1900i ultraviolet-visible (UV-Vis) spectrophotometer and TMSPC-8 Tm analysis system. This system provides a simple method to calculate the Tm value by measuring the absorbance transition at 260 nm at increasing temperatures.
Learning outcome:
Discover how the TMSPC-8 Tm analysis system can simplify thermal stability analysis of nucleotides.
Synthesis Confirmation for Nucleic Acid Medicines - Rapid Sequence Confirmation Using a MALDI-TOF Mass Spectrometer This application shows how the molecular weight and base sequence of a synthetic nucleic acid can be determined using MALDI-TOF mass spectrometry.
This application shows how the molecular weight and base sequence of a synthetic nucleic acid can be determined using MALDI-TOF mass spectrometry.
Learning Outcome:
See how Shimadzu’s benchtop MALDI-8020 mass spectrometer makes it easy to confirm the molecular weight and base sequence of nucleic acids.
Verification of Quantitativity and Confirmation of Molecular Weight of Oligonucleotide Therapeutics Using LCMS™-8060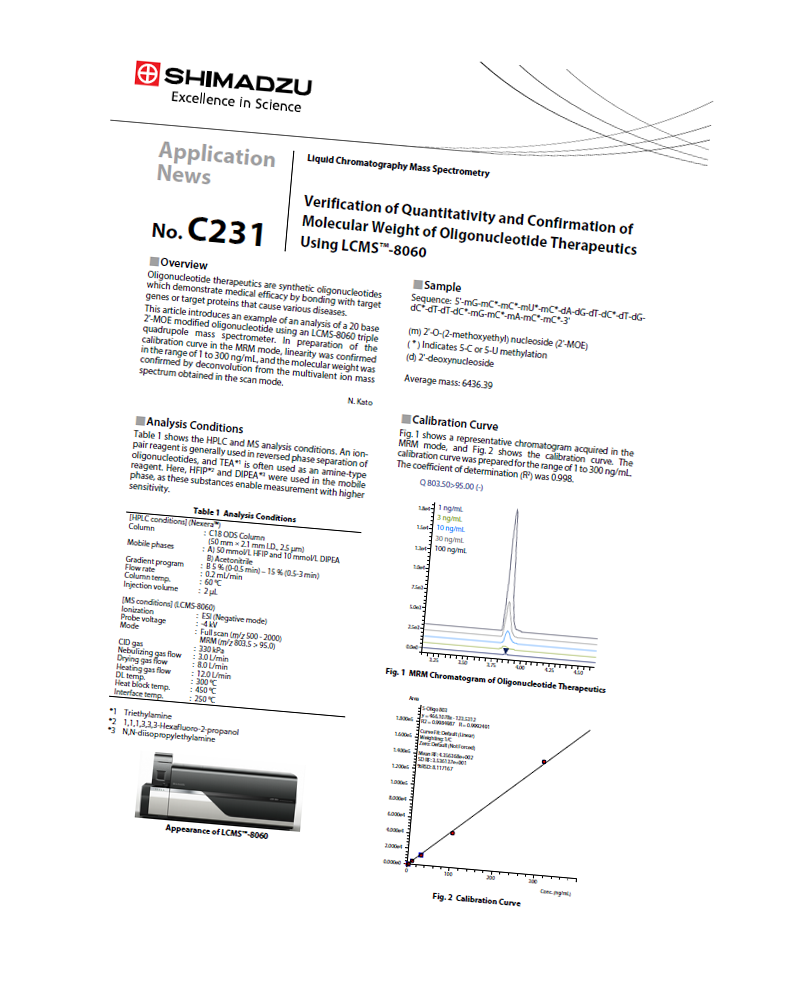 Here we introduce the analysis of a 20 base 2’-MOE modified oligonucleotide using an LCMS-8060 triple quadrupole mass spectrometer. A calibration curve for 1 to 300 ng/mL was obtained, and the molecular weight was confirmed within a small error range by deconvolution function from the scan data.
Here we introduce the analysis of a 20 base 2’-MOE modified oligonucleotide using an LCMS-8060 triple quadrupole mass spectrometer. A calibration curve for 1 to 300 ng/mL was obtained, and the molecular weight was confirmed within a small error range by deconvolution function from the scan data.
Learning Outcome:
Learn how Shimadzu’s LCMS-8060 can be used to determine the molecular weight of synthetic oligonucleotides.
Determination of Molecular Mass and Quantification of Oligonucleotide Therapeutics Using Quadrupole Time-of-Flight Mass Spectrometer LCMS™-9030 This application shows an example analysis of a 20 base 2’-MOE modified oligonucleotide using the LCMS-9030 Q-TOF mass spectrometer. The molecular mass was determined with an error of just 0.05 ppm. A calibration curve was prepared using the MRM mode, demonstrating linearity within a range of 1–1000 ng/mL.
This application shows an example analysis of a 20 base 2’-MOE modified oligonucleotide using the LCMS-9030 Q-TOF mass spectrometer. The molecular mass was determined with an error of just 0.05 ppm. A calibration curve was prepared using the MRM mode, demonstrating linearity within a range of 1–1000 ng/mL.
Learning outcome:
Learn how the accurate mass measurement of Shimadzu’s LCMS-9030 can be used to identify the structure of oligonucleotide therapeutics.
Don't miss out on the latest COVID-19, nitrosamine analysis or LC/MS bioanalysis of antibody drugs applications. Click below for PDFs.
Click here to access the COVID-19 app notes >>
Click here to access the nitrosamine analysis app notes >>
Click here to access the LC/MS bioanalysis of antibody drug app notes >>




