Metrohm has produced an application note demonstrating a method for a zinc oxide assay as per USP General Chapter <591>.
 USP is updating the General Chapter <591> «Zinc Determination» monograph to include ion chromatography as a method for the assay. Zinc oxide is used in various skin care creams, drugs, and drug products. The ion chromatography analysis involves separation of zinc followed by post-column reaction using 4-(2-pyridylazo) resorcinol (PAR) reagent and subsequent detection at
USP is updating the General Chapter <591> «Zinc Determination» monograph to include ion chromatography as a method for the assay. Zinc oxide is used in various skin care creams, drugs, and drug products. The ion chromatography analysis involves separation of zinc followed by post-column reaction using 4-(2-pyridylazo) resorcinol (PAR) reagent and subsequent detection at
530 nm wavelength.
Sample preparation consisted of 0.1868 g of sample dissolved in 10 mL 6 mol/L HCl and made up to 100 mL with ultrapure water.
Columns used for this application were Metrosep A Supp 10 - 250/4.0 6.1020.030 and Metrosep A Supp 10 Guard/4.0 6.1020.500.
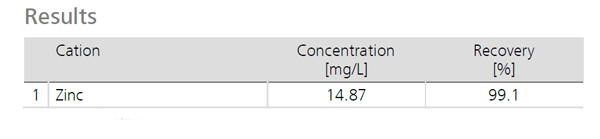
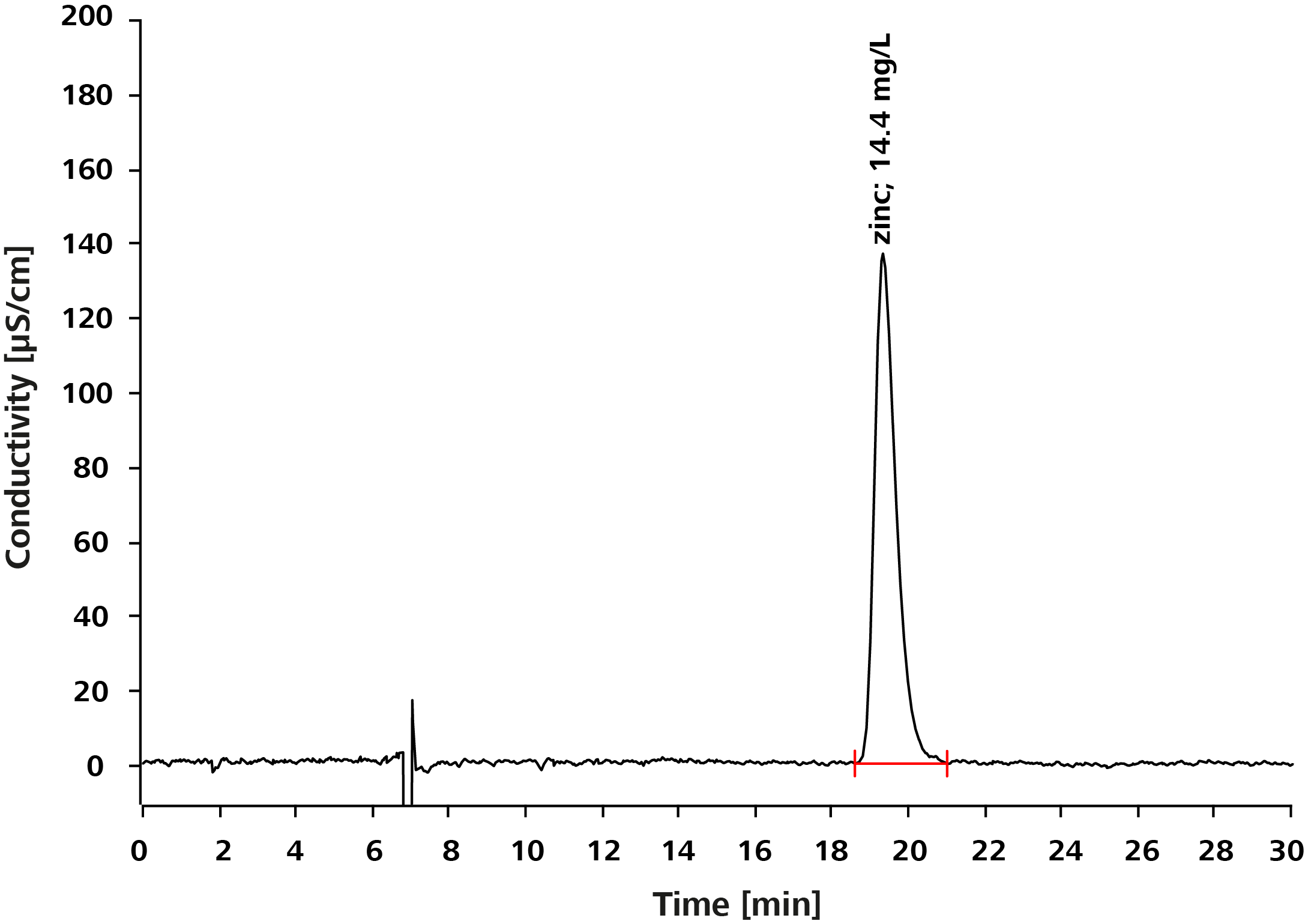
Results can be viewed below.